
Novel Profiling of Influenza Immune History: Implications on Vaccine-Induced Immunity and Efficacy
Introduction
Influenza is responsible for hundreds of thousands of deaths every year and imposes significant health and economical burdens. The rapid evolution of influenza viruses has prevented the development of a long-lasting universal vaccine, thus requiring an annual vaccination. Influenza vaccination has varying protective efficacies, particularly in young children and the elderly, which have a higher risk of developing severe disease. Throughout life, individuals are repeatedly exposed to influenza viruses through infections and vaccinations, leading to the development of immunological memory consisting of both T and B-cells. This immunological history–is one of the major contributors to the variety of responses to influenza vaccines. Assays that will improve the ability to study and predict immune responses to influenza infections and vaccination are highly needed and can aid in developing better vaccines.
The Technology
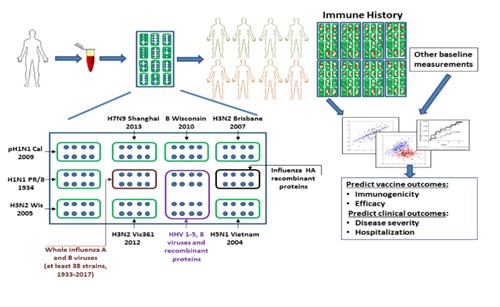
We have developed a novel, sensitive and cost-effective antigen microarray, which will enable profiling of the antibody repertoire to influenza vaccination and natural infection. Our antigen array contains influenza –derived peptides, recombinant surface proteins and whole viral particles. Using serum samples from animals and humans, we generated influenza antibody profiles measured at baseline and post-vaccination and used these to predict vaccine-induced immune responses and outcomes such as vaccine efficacy and disease severity. To generate these arrays, we are screening thousands of potential antigens and develop statistical tools to identify a minimal set of antigens that can be used to profile influenza immunological history. A technological proof of concept was already established. Clinical POC on human patients due to start Q3 2021.
Advantages
- Novel approach for influenza immune history profiling
- User-friendly software that allows robust analysis of the results for biologists will be developed
- Optional tailoring for different sub-populations and experimental designs
Patent Status
Application was filed on December 8th 2020, under the application number PCT/IL2020/051270
Partnering
Co-development or licensing out for further development
Principal Investigator
Prof. Tomer Hertz, NIBN and the Shraga Segal Department of Microbiology, Immunology and Genetics, Ben-Gurion University of the Negev, Israel
